

More realistic treatments of varying particle viscosity with changing RH indicate these timescales could decrease to micro-seconds at high RH due to the plasticising effect of water 18.
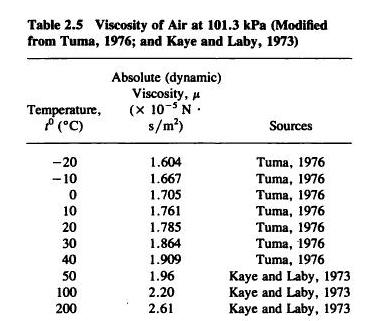
Indeed, single particle models initially suggested that mixing timescales could increase to anywhere from >1 h to 1 year depending on the particle size 17. Such a lengthening has been used to propose significant kinetic limitations on growth and evaporation (Fig. Einstein first established the slowing of diffusional motion in a medium of increasing viscosity, with this inverse relationship apparent in the Stokes–Einstein equation: By contrast, highly viscous organic particles can be slow to respond to changes in gas-phase composition, particularly under dry conditions or at low temperatures 5, 7, 13. Liquid organic particles with low viscosity are responsive to changes in gas-phase composition and take up or lose water in response to variations in ambient relative humidity (RH) and temperature 13, 14. As an example, the rates of growth and evaporation of organic particles are dependent on particle viscosity, with direct implications for climate, visibility and air quality 10, 11, 12. The viscosity of atmospheric OA is central to rationalising and predicting their atmospheric impacts (Fig.
Thus, much of our review necessarily considers the consensus that is emerging from laboratory work on the phase state of ambient particles, supported by the limited number of field measurements that are now emerging. Many of the studies on which our understanding of atmospheric aerosol particle viscosity is based have been undertaken in the laboratory using surrogates of ambient particles this is a consequence of the challenges of making aerosol particle viscosity measurements directly. Our focus here is to explore the compositional and environmental factors that govern particle viscosity, the consequences of particle viscosity for aerosol microphysics and the global impacts that can then result. In stark contrast, recent measurements of organic particle properties often imply the existence of highly viscous semi-solid and even amorphous solid particles 4, 5, 6. Until recently, researchers assumed that atmospheric organic particles are liquid in phase and, hence, have low viscosity. The composition of SOA is especially uncertain, with only approximately 10% of the mass of secondary OA identified at the molecular level 2.
#DYNAMIC VISCOSITY AIR SURFACE SERIES#
Ambient organic aerosols (OA) can be emitted directly (referred to as primary OA) or can be formed by a complex series of reactions (referred to as secondary OA, SOA) 3. The organic component can represent 50% or more of the mass of the fine aerosol particle fraction (particles smaller than 1 μm in diameter) in the atmosphere 2. Atmospheric particles, which range in size from <10 nm to ∼10 μm, consist of both inorganic and organic material 1, 2.


 0 kommentar(er)
0 kommentar(er)
